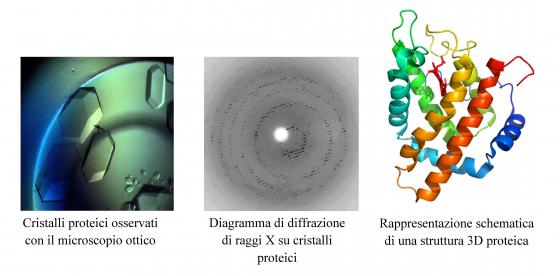
3D-protein structure determination through X-ray diffraction on crystals and solution specimens
The research activity of this research line is focused on the 3D structure determination and analysis of macromolecules and their complexes with other proteins, and/or substrates/inhibitors using X-ray diffraction (on crystal and solution specimens), computer modeling and molecular simulation techniques. The protein crystals are grown in the fully equipped wet-lab located at the Physics Dept. such as the diffraction data analysis and structure determination using specific software programs.
The X-rays diffraction experiments on protein crystals are carried out at European Synchrotron Facilities (ESRF, Grenoble, France), while the experiments on protein solutions (Small Angle X-ray Scattering, SAXS) at DESY synchrotron (Hamburg, Germany).
The main research line is focused on 3D structure determination at high resolution of hemeproteins found in plants, invertebrates and bacteria and on low resolution structure of early aggregation states of amyloidogenic proteins.
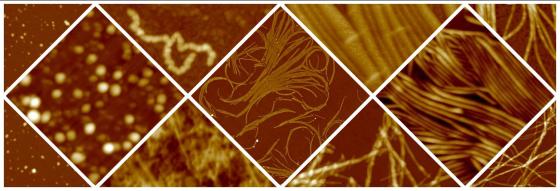
Protein aggregation and amyloid fibrils
In a number of severe diseases, including Alzheimer's and Parkinson's diseases, a process of incorrect folding, or misfolding, of the polypeptide chain occurs. This leads to the formation of fibrillar aggregates, called amyloid fibrils, which have a diameter of a few nanometers and a length of the order of a micrometer. Understanding the molecular details of the aggregation process and the mechanisms involved in aggregate toxicity is important for the development of therapeutic strategies. More recently, physiological processes involving the controlled formation of amyloid fibrils have been discovered. Finally, these structures represent a potentially interesting material for the construction of nanowires or other nanostructured devices for nanotechnological applications.
The aim of our research activity is to understand the molecular mechanisms of the aggregation process and to identify the factors that influence it, characterizing the physical properties of the aggregates through techniques such as scanning probe microscopy and spectroscopy, fluorescence spectroscopy, diffusion of light, small angle X-rays scattering. With this approach we are also able to study the effects of aggregation-inhibiting agents. From the study of aggregate interaction with biomimetic membranes such as Langmuir films, lipid double layers on solid support and liposomes, information can be obtained on the mechanisms of membrane destabilization by protein aggregates.